NMR spectroscopy basics
 Protons spin and they have charge. According to Maxwell's equation, protons
should be little magnets and they are. If you have used a compass, you know that a
little magnet (the compass needle) will line up with a magnetic field. Protons do the
same. However, unlike compasses, protons are constrained by quantum mechanics. They can
only be parallel or anti-parallel to the magnetic field. When they flip from one state
to the other they emit or absorb a photon. The frequency of the photon depends directly
on the strength of the magnetic fields. The frequency is called the Larmor precession
frequency and it is in the range of a short wave radio. This is also the basis of
magnetic resonance imaging (MRI.) It should be called "NMRI" but some people don't like
that word "nuclear."
Protons spin and they have charge. According to Maxwell's equation, protons
should be little magnets and they are. If you have used a compass, you know that a
little magnet (the compass needle) will line up with a magnetic field. Protons do the
same. However, unlike compasses, protons are constrained by quantum mechanics. They can
only be parallel or anti-parallel to the magnetic field. When they flip from one state
to the other they emit or absorb a photon. The frequency of the photon depends directly
on the strength of the magnetic fields. The frequency is called the Larmor precession
frequency and it is in the range of a short wave radio. This is also the basis of
magnetic resonance imaging (MRI.) It should be called "NMRI" but some people don't like
that word "nuclear."
The Varian A60 NMR spectrometer
 Click here to see why
the A60 was considered a "landmark" instrument. It was introduced in 1961 and ultimately
1000 were sold. The "60" comes from 60 megahertz, the Larmor frequency the A60 operated
at. NMR signals are often weak, but their strength is proportional to the magnetic field
raised to the 3/2 power. So, you want a strong magnet. The magnetic field also has to be
very uniform. These are the reasons why the iron magnet in the A60 weighs more than an
SUV and consumes $20,000 worth of electricity each year.
Click here to see why
the A60 was considered a "landmark" instrument. It was introduced in 1961 and ultimately
1000 were sold. The "60" comes from 60 megahertz, the Larmor frequency the A60 operated
at. NMR signals are often weak, but their strength is proportional to the magnetic field
raised to the 3/2 power. So, you want a strong magnet. The magnetic field also has to be
very uniform. These are the reasons why the iron magnet in the A60 weighs more than an
SUV and consumes $20,000 worth of electricity each year.
The A60 worked in the following way: You put the sample to be analyzed into the maw of the magnet. The A60 had a radio transmitter and a radio receiver, both tuned to the Larmor frequency. The flipping protons affect the coupling between the receiver and the transmitter but only if the magnetic field corresponds to the Larmor frequency exactly. This happens at several different field strengths because of the chemical shifts. The magnetic field is swept until all the chemical shifts are plotted. Each scan takes minutes. This approach is called carrier wave NMR or CWNMR.
The A60 and spectrometers like it had a serious drawback—noise. However, signal averaging could be used to improve the signal to noise ratio. The Mnemotron and later a Nicolet signal averager were used for this. Signal averaging required patience it took hours to get a result. Signal averaging often meant scanning overnight.
It fell upon me, in 1967, to connect a Fabritek (later Nicolet) 1050 to an A60 in the field. I had never seen one before. Bob Schumann designed the interface and the plugin. I was supposed to find the right signals inside the A60 by perusing the schematics with the customer looking on. It was clear I had failed when the A60 smoked. Nobody told me about a connector on the back of the A60 labeled "CAT1024" with all the correct signals brought to it. The CAT1024 connector was meant for the extinct Mnemotron.
More NMR spectroscopy basics
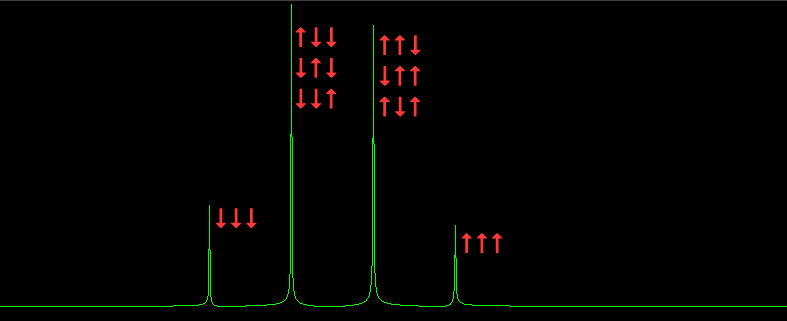
If two protons are close together, both are affected by the others magnetic field. This is called the chemical shift. Physicists were annoyed by chemical shifts, but chemists quickly realized that chemical shifts could be used to determine the structure of molecules. That is because a bare proton is the nucleus of a hydrogen atom. Virtually all interesting molecules have hydrogen atoms if you are a biochemist. Bare in mind that chemical shifts are millions of times smaller than the Larmor precession frequency. Here is a graph that shows what happens when one proton is in the magnetic fields of 3 other protons all the same distance away. The arrows show the 8 possible ways the spins can add or subtract. Note the 1,3,3,1 amplitude ratios of the quartet corresponding to the spin states.
The A60 was a proton-only machine. But it is possible to do NMR with other nuclei. Any nucleus with either an odd number of protons or neutrons will work. Biochemists would especially like to do NMR with carbon, but they can't because quantum mechanics says they can't. The spins of the 6 protons exactly cancel, and the spins of the 6 neutrons of the carbon nucleus exactly cancel which means no little magnets and, therefore, no NMR. But there is a rare isotope carbon called carbon 13, which has an extra neutron and so it is a little magnet. This fact about carbon 13 gets the attention of biochemists. Unfortunately, the carbon 13 NMR signal is weak due to the rarity of the isotope. Biochemist also have compelling reasons to do NMR with deuterium, fluorine, nitrogen too. The next generation NMR spectrometers would have to be multinuclear.
The central problem was noise. If only there was a way to continuously collect information from the entire spectrum.
FTNMR spectroscopy basics
The radio transmitter in a CWNMR has a fixed frequency and so it irradiates only one element of the NMR spectrum at a time. But it is possible to irradiate the entire NMR spectrum by pulsing the radio transmitter. The pulse is strong, but narrow, on the order of a millisecond. It is like whacking a piano with a baseball bat to make all the strings sound simultaneously. The magnetic field is not swept. The radio receiver listens after each pulse. It "hears" all of the chemically shifted Larmor frequencies simultaneously. It is possible to plot the result and it looks like this:
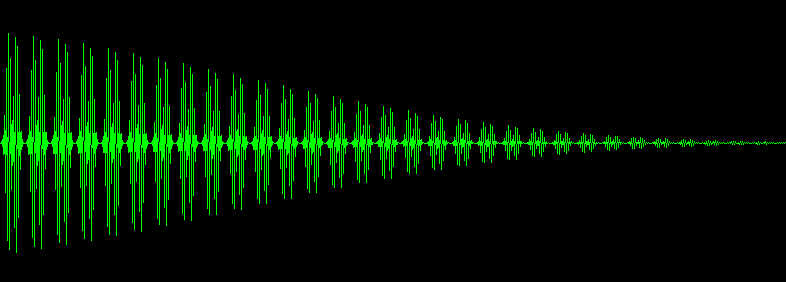
This thing has a name. It is called a Free Induction Decay or a FID. Note that the horizontal axis is labeled "frequency" in the first case and "time" in the second. A FID contains information collected from the entire NMR spectrum. That means the signal to noise ratio has been improved by one or two orders of magnitude. This is called Fellgett's advantage. More about Fellgett's advantage here. However, there is a problem; the data are not in a usable form.
Here comes the "aha" moment. The Fourier transform of the FID is the NMR spectrum! The 1,3,3,1 NMR spectrum was obtained by Fourier analysis of this particular FID. The FFT program that actually produced this FID is elsewhere on this site. This FID is not a real NMR spectrum. It was contrived for the purpose of illustration.
It is possible to connect the radio receiver to a loudspeaker and listen to FIDs. The above FID sounds like this. The sound of FIDs has come to be known as a kabong. It is sometimes possible to identify samples by listening to their kabongs. Musicians might be especially good at this. Perhaps chemists should hire musicians instead of buying computers. Maybe in a parallel universe, where Cooley and Tukey never existed, musicians have good paying jobs as kabong analyzers.
 Richard R. Ernst had this "aha" moment in 1966. He was awarded the 1991 Nobel prize in
chemistry for it. You can read the press release on the Nobel Prize website here. The
press release may be read on this site here. FTNMR
is also the foundation of Magnetic Resonance Imaging (MRI). Ernst's method of doing
FTNMR required a truck. The computer, a room sized IBM 7090, was located several miles
from the spectrometer. The only way to get the FID into the IBM 7090 was
punchcards—a truck load of punchcards. The IBM 7090 went for $3,000,000 and memory
for it was a dollar a bit(!) in 1966—in 1966 dollars. It was not hard to sense a
business opportunity here.
Richard R. Ernst had this "aha" moment in 1966. He was awarded the 1991 Nobel prize in
chemistry for it. You can read the press release on the Nobel Prize website here. The
press release may be read on this site here. FTNMR
is also the foundation of Magnetic Resonance Imaging (MRI). Ernst's method of doing
FTNMR required a truck. The computer, a room sized IBM 7090, was located several miles
from the spectrometer. The only way to get the FID into the IBM 7090 was
punchcards—a truck load of punchcards. The IBM 7090 went for $3,000,000 and memory
for it was a dollar a bit(!) in 1966—in 1966 dollars. It was not hard to sense a
business opportunity here.